|
|||||
|
Proc.
6th International Congress of Genetics pp. 343-355 (1932)
THE
NATURE OF SEX CHROMOSOMES
O. Winge, Copenhagen, Denmark
On the whole, it may be said that the sex difference between males and females is of genotypical nature, and that the primary sex determination is dependent on sex chromosomes.
We distinguish between the two classical main types, first set up by E. B. WILSON: (1) the Protenor type, with 2 X chromosomes in individuals of one sex and 1 X chromosome in the other; and (2) the Lygaeus type, with 2 X chromosomes in one sex, whereas the other has 1 X and 1 Y chromosome.
As is well known, in some classes the male is heterogametic, while in others the female has this peculiarity; and in the latter case the sex chromosomes are often called Z and W, instead of being designated as X and Y. In passing, I wish to say it does not seem reasonable to complicate matters by using sometimes the designation X-Y, and sometimes Z-W. This distinction, it seen-is to me, can serve no other purpose than that of "making it harder." I wish to suggest that the designations X and Y always be used.
The fact that the males and females of the Protenor type differ only in this respect, that the male has 1 X while the female has 2 X, establishes that unquestionably we are here dealing with quantitative, and not qualitative, differences of the two sexes. Furthermore, the Drosophila experiments have demonstrated that a similar view applies to this species too. The number of X, in proportion to the number of autosomes, is the decisive factor in the sex determination of the offspring.
The more the researches into the field of heredity progress, the more convincing is the evidence that the nature of the individual, implying its sex, is dependent upon the preponderance of some gene, or genes, in proportion to the others present. As already emphasized by BRIDGES, each faculty of the individual will be dependent, at least to some degree, upon the proportion of balance between the genes, those that accentuate and those that attenuate the establishment of a given property. Finally, the hypothesis advanced by GOLDSCHMIDT on the significance of the gene quantities to the properties of the individual, including the sex characteristics, is quite consistent with the view that the nature of the organism as a whole is determined by the balance between all the genes inherent in the organism.
In this lecture the aim is to demonstrate, partly through facts that have already been published, not only that cytological observations and genetic experiments substantiate the view that the sex chromosomes are to be regarded as originating from autosomes, but also that it is practicable experimentally to alter X chromosomes into autosomes and autosomes into X and Y chromosomes; and, finally, it will be shown that under certain conditions the difference between X and Y is merely of quantitative nature.
As a rule, indeed, the X chromosome and the Y chromosome differ morphologically, though this is not always the case. In the fish Lebistes reticulatus, belonging to the Poeciliidae, it is not possible to distinguish between X and Y, and crossing over is often taking place. When, nevertheless, X and Y keep maintaining their respective nature, the explanation of this is to be looked for undoubtedly in the fact that these two chromosomes differ only on one point, in a single gene: the Y chromosome contains an epistatic male-determining gene, which is not present in the X chromosome. Even after crossing over between X and Y, only one of the chromosomes will contain the male gene, and this chromosome does thus become a Y chromosome.
It is probable that, in analogy with the conditions in Drosophila, in Lebistes, too, there are several other genes which might tend to influence the sex of the offspring. But the Y chromosome contains a dominant and epistatic male-determining gene which, under ordinary conditions, constitutes the sole decisive factor in the establishment of the male sex.
Some geneticists have a different view, on the sex-determination of Lebistes, especially WITSCHl, who thinks it is just as in Drosophila. I regret I have no time for discussing this today. I only want to add that I find it too dogmatic to assume that all organisms behave just as Drosophila do. In Lebistes you have more color genes in the Y than in any other chromosome, and you find crossing over between X and Y. The last fact makes it obvious that you have a special gene or gene-pair for the sex in the sex chromosomes or, as proposed by MORGAN, a special part of the sex chromosomes has the sole sex-determining effect.
In a dioecious plant such as hop, Humulus lupulus, we find a typical pair of XY chromosomes in the male, and a pair of XX in the female. Here the Y chromosome is twice as large as X. Besides, the X chromosome is somewhat constricted in the middle, especially during the prophase prior to the reduction division.
A priori one might think, perhaps, that in this species X and Y differ in quality, as they look entirely different. In reality, however, there can be no doubt that the Y chromosome is equal to two X chromosomes which have become united.
On microscopic examination of the reduction division (figure 1) in the male plant of the near-related Humulus Japonicus, one may find, even in the same field of view, now an XY pair of chromosomes where Y is twice as large as X, now a Y chromosome that is constricted in the middle, and now 3 chromosomes of the same size, that is, 3 X chromosomes. And sometimes the reduction division proceeds in this way: the median sex chromosome goes to one pole while the two terminal sex chromosomes go to the other pole; sometimes one terminal chromosome goes to one pole, while the two adjoining chromosomes go to the other pole. In brief, one has a very distinct impression that in Humulus Japonicus the Y chromosome is equal to 2 X chromosomes. Thus, in this species too the sex determination is dependent upon unequal quantities of sex-determining genes, upon a difference in the proportional balance between the genes with a female tendency and those with a male tendency.
 |
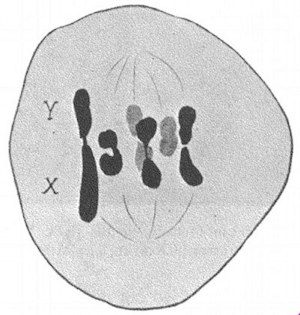 |
| FIGURE 1.—Variation in the sex chromosome complement at the reduction division in the male Humulus Japonicus. | FIGURE 2.—The sex chromosome pair in the male Melandrium rubrum. The X is twice as large as the Y. |
There can be no doubt then that also in Humulus lupulus the Y chromosome is composed of two X chromosomes, conjugated end to end. The difference between the two species of Humulus is merely this: in one of the species, evidently, the X chromosomes are conjugated permanently into one unit, the so-called Y chromosome.
In Melandrium album and M. rubrum the male plant shows a quite typical pair of XY chromosomes, but here the X chromosome is twice as large as Y (figure 2). It is true that up to the present it has not been practicable to demonstrate cytologically that X is composed of 2 Y chromosomes; yet, the genetic experiments I have carried out with this plant leave hardly any question of this being the case.
 |
| FIGURE 3.—A normal Melandrium (female) to the right. An "abnormal" to the left. The "abnormal" gene, n, may be present in X or in Y in Melandrium. |
Amongst the X linked and Y linked genes I have been able to demonstrate in Melandrium (the demonstration material is represented here in the exposition field) there is a recessive gene I have called "abnormal" (figure 3). This gene may be present in both the X and the Y chromosome, and it is inherited in a perfectly regular manner, either X linked or Y linked (figure 4).
How, one may ask, is the presence of this characteristic gene in either kind of sex chromosomes to be explained? The assumption that the Melandrium X is composed of 2 Y chromosomes (figure 5) offers a very simple explanation of this. Occasionally, on bipartition of X during the prophase of the reduction division in a pollen mother cell, the result will be 3 free Y chromosomes, in "end-to-end" position, and the original Y may probably enter into a new-formed X. The free half of the X chromosome, on the other hand, constitutes itself a new Y chromosome. It is a special case of translocation.
 |
| FIGURE 4.—By crossing an abnormal female Melandrium (XnXn) with a heterozygous normal male, entirely different results are obtained in proportion as the "abnormal" gene is present in the X or in the Y of the male. In the first case all daughters are abnormal, all sons normal. In the second case the opposite result is found. |
Just as the X chromosomes and the Y chromosomes are here proved to be of identical nature, so I have succeeded in experiments with Lebistes in altering the sex chromosomes into autosomes and vice versa.
The male Lebistes has a pair of X-Y chromosomes that cannot be distinguished one from the other, nor can they be distinguished from the autosomes (there are, all told, 23 pairs of chromosomes). Still, the fact that nearly all the genes which give to the Lebistes males their splendid color schemes are inherited as X linked or Y linked is an indisputable evidence of the presence of sex chromosomes.
 |
| FIGURE 5.—Illustrating the presence in Melandrium of the same recessive gene, n (for "abnormal"), in both X and Y, X probably being composed of two Y chromosomes. |
It is only the Lebistes males (figures 6 and 7) that show color patterns, whereas the females are continuously greyish even though they have some genotypical faculty for coloring. In the males the color genes are dominant, and the recessive allelomorphs are absent. Here the "presence-absence theory" holds good. Figure 8 shows the combined X and Y linked inheritance of 5 genes, Coccineus, Vitellinus, Tigrinus, Luteus and Maculatus.
In a couple of the races I have experimented with, however, it has not been an altogether uncommon finding that individuals have resulted that are essentially females, though they have some tendency for the formation of a gonopod, the copulation organ characteristic of the males, and at the same time they have shown the color patterns corresponding to their genetic formula.
This applies in particular to the two races characterized respectively by the genes Coccineus-Vitellinus and Tigrinus-Luteus in the X chromosomes, which races have shown a rather marked tendency to produce such masculinized females (see figure 9).
 |
| FIGURE 6—The effect of different color genes in Lebistes males. Vertical hatching indicates red color, while dotting indicates yellow color. |
In order to try whether it might be possible to produce females entirely masculinized, I have made some crossings between these two races. A homozygous female XCo,Vi XCo,Vi was crossed with a male XTi,Lu YMa (figure 9). The "Ma" gene in the male Y chromosome designates the gene "Maculatus" which is linked absolutely to the Y chromosome, most likely because it is identical with the very male-determining gene in Y. It has never shown any crossing over to the X chromosome, not even amongst many thousands of individuals. All the male offspring of a male Maculatus show the male Maculatus gene—for one thing, they have the characteristic black spot in the dorsal fin.
 |
| FIGURE 7.—For explanation see figure 6. |
The crossing experiments gave, as was to be expected, chiefly males of the formula XCo,Vi YMa (55 individuals) together with a corresponding number of colorless, or nearly colorless, females; but, in addition, there appeared 3 males, showing the genes Co, Vi and Ti, Lu, whereas they were wanting Ma (the type is shown on figure 9, below, in the middle). Obviously, these exceptional males are XX males, for if they had any Y chromosomes they would present the Maculatus gene. They had merely the color pattern the females would have possessed if the latter had been able to bring forth their content of color genes.
Now it could be predicted, and it was predicted, that these exceptional males, which were bound to he XX individuals, would give exclusively female offspring when they were mated with ordinary females. In fact, this proved to be the case. All told, the 3 XX males produced a total of 314 young, and every one of the young was a female; there was not a single male in the lot, while in all other cases I used to have about 50 percent of each sex.
 |
| FIGURE 8.—A combined X and Y linked inheritance of color genes in Lebistes. |
To obtain anew some XX males there was only one way open: backcrossing some of the many daughters with the XX males (figure 10). On such backcrossing, one of them gave 164 young. And they were all females too. But one of these 164 daughters was again backcrossed with the XX male, and now it gave 30 females and I male, which proved to be a XCo,Vi XTi,Lu.
 |
| FIGURE 9.—Two upper rows: The single effect in the Lebistes male of the genes Coccineus, Vitellinus, Maculatus, Luteus and Tigrinus, and a half-masculinized female showing colors according to her formula. |
Two lower rows: A cross between the CoVi-race and the TiLu-race, giving chiefly CoViMa-males, as expected, but, in addition, a male type with CoViTiLu, that is, the female formula, with two X chromosomes and no Y.
This new XX male was backcrossed with its mother, and now the outcome was males and females in equal number, though not so many that it was warrantable to give their sex ratio as exactly 50 percent of either sex. (Addition November 1932.) Counting together, however, all the material showing segregation in males and females in the new XX race, 37 males and 34 females are obtained, namely 5:2, 4:5, 3:3, 9:4 and 16:20, respectively. If the XX males are crossed to females of a normal race, only daughters are obtained.
 |
| FIGURE 10.—A Lebistes XX male crossed to an ordinary female gives only daughters (101). Backcrossing one of the daughters to the XX male gives again only daughters (164). Backcrossing one of these to the XX male gives 30 females and 1 male. By backcrossing this male to his mother, males and females are segregated, apparently in a 1:1 ratio, and the X linked genes are now inherited in normal Mendelian manner. For further explanation see text. |
As will be recognized readily, this means the establishment of a new sex determination. Now the XX chromosomes are to be looked upon merely as ordinary autosomes, identical for individuals of either sex. Accordingly, the genes Coccineus, Vitellinus, Tigrinus and Luteus are inherited in ordinary Mendelian manner, without being sex linked, and they have no connection whatever with the sex determination. The X chromosomes are modified into autosomes.
 |
| FIGURE 11.—Explanation of the transition from the normal Lebistes with XX in females and XY in males, to the new type, with Aa in females and aa in males (both sexes having now XX). In this new type A must be regarded as a new "Y" chromosome, and a as a new "X". The formulas for the ordinary Lebistes, as distinguished from the CoVi and TiLu races, have about the same number of autosomal genes pulling in male and in female direction, while the CoVi and TiLu races are supposed to have a surplus of autosomal genes pulling in male direction. By accidental accumulation of the male genes in certain individuals the development may be in male direction even in XX individuals. |
Nevertheless, the mechanism of sex determination is brought forth through the outlined backcrossing to XX males, whereby a new pair of chromosomes have taken over the leading role in the sex determination, a pair of chromosomes which were of no particular significance in this respect as long as the ordinarily effective function of the differentiating XX-XY mechanism was maintained.
On backcrossing to XX males there is a selection of autosomal genes which have a male-determining tendency; and, theoretically, there is no reason why female heterogamy should not result, so that, in future, the difference between the females and the males is that the females are heterozygous (Aa) as to a female determining gene A, while the males are of the homozygous recessive formula (aa), both sexes having XX. It is obvious that this will result in a new sex linked (X and Y linked) inheritance of genes in the A-a chromosome pair (figure 11).
However, at present it is impossible to establish whether, in this new pair of chromosomes, the male or the female is the heterogametic. A priori, the chances are about equal.
In this way it is evident that it is possible, within a given species, to cross from male to female heterogamy. Thus it is less surprising that Lebistes shows male heterogamy, while the near-related Platypoecilus shows female heterogamy, as has been pointed out by BELLAMY (1922), by GORDON, and others.
Naturally, it is to be expected, when the sex-determining genes of lower order take on the leading role, there will exist the possibility that external conditions may influence the sex determination. In the near-related Xiphophorus Helleri, indeed, the sex determination appears to be very uncertain, so uncertain, even, that it has been claimed it is entirely phenotypical. I know, however, from my own experience with Xiphophorus that this is carrying the point to excess.
It has been my main attempt to show how the view concerning quantitative differences may be applied at least in some cases on the X and the Y chromosomes, and to show that even the difference between sex chromosomes and autosomes, as to their sex-determining ability, is of quantitative nature, and that sex chromosomes experimentally can be changed into autosomes and vice versa.
Gordon, M. 1952. Genetics of Xiphophorus (Platypoecilus) maculatus. III. Differentiation of gonads in platyfish from broods having a sex ratio of three females to one male. Zoologica (N.Y.) 37: 91-100.
| Copyright 2022 Richard J. Sexton |